Substrate recognition mechanism of a glycosyltrehalose trehalohydrolase from 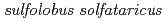 KM1
KM1
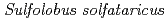 KM1由来グリコシルトレハローストレハロハイドロレースの基質認識機構の解明
KM1由来グリコシルトレハローストレハロハイドロレースの基質認識機構の解明
岡崎 伸生; 玉田 太郎; Feese, M. D.*; 加藤 優*; 三浦 裕*; 米田 俊浩*; 小林 和男*; 近藤 恵二*; Blaber, M.*; 黒木 良太
Okazaki, Nobuo; Tamada, Taro; Feese, M. D.*; Kato, Masaru*; Miura, Yutaka*; Komeda, Toshihiro*; Kobayashi, Kazuo*; Kondo, Keiji*; Blaber, M.*; Kuroki, Ryota
Glycosyltrehalose trehalohydrolase (GTHase) is an  -amylase that cleaves the
-amylase that cleaves the  -1,4 bond adjacent to the
-1,4 bond adjacent to the  -1,1 bond of maltooligosyltrehalose to release trehalose. In order to investigate the catalytic and substrate recognition mechanisms of GTHase, two residues, Asp252 (nucleophile) and Glu283 (general acid/base), located at the catalytic site of GTHase were mutated (Asp252
-1,1 bond of maltooligosyltrehalose to release trehalose. In order to investigate the catalytic and substrate recognition mechanisms of GTHase, two residues, Asp252 (nucleophile) and Glu283 (general acid/base), located at the catalytic site of GTHase were mutated (Asp252  Ser (D252S), Glu (D252E) and Glu283
Ser (D252S), Glu (D252E) and Glu283  Gln (E283Q)), and the activity and structure of the enzyme were investigated. The E283Q, D252E and D252S mutants showed only 0.04%, 0.03%, and 0.6% of enzymatic activity against the wild-type, respectively. The crystal structure of the E283Q mutant GTHase in complex with the substrate, maltotriosyltrehalose (G3-Tre), was determined to 2.6
Gln (E283Q)), and the activity and structure of the enzyme were investigated. The E283Q, D252E and D252S mutants showed only 0.04%, 0.03%, and 0.6% of enzymatic activity against the wild-type, respectively. The crystal structure of the E283Q mutant GTHase in complex with the substrate, maltotriosyltrehalose (G3-Tre), was determined to 2.6  resolution. The structure with G3-Tre indicated that GTHase has at least five substrate binding subsites and that Glu283 is the catalytic acid, and Asp252 is the nucleophile that attacks the C1 carbon in the glycosidic linkage of G3-Tre. The complex structure also revealed a scheme for substrate recognition by GTHase. Substrate recognition involves two unique interactions: stacking of Tyr325 with the terminal glucose ring of the trehalose moiety and perpendicularly placement of Trp215 to the pyranose rings at the subsites -1 and +1 glucose.
resolution. The structure with G3-Tre indicated that GTHase has at least five substrate binding subsites and that Glu283 is the catalytic acid, and Asp252 is the nucleophile that attacks the C1 carbon in the glycosidic linkage of G3-Tre. The complex structure also revealed a scheme for substrate recognition by GTHase. Substrate recognition involves two unique interactions: stacking of Tyr325 with the terminal glucose ring of the trehalose moiety and perpendicularly placement of Trp215 to the pyranose rings at the subsites -1 and +1 glucose.