Neutron scattering study on dynamics of hydration water around muscle contractile proteins
中性子散乱による筋収縮タンパク質水和水のダイナミクス解析
松尾 龍人; 荒田 敏昭*; 小田 俊郎*; 藤原 悟
Matsuo, Tatsuhito; Arata, Toshiaki*; Oda, Toshiro*; Fujiwara, Satoru
筋肉収縮は、F-アクチンとミオシンというタンパク質の相互作用によって生じる。この相互作用は、溶媒の熱揺らぎをタンパク質が積極的に利用して行われると考えられている。溶媒分子の中でも特に、水和水の存在がタンパク質の正常な機能発現に必要不可欠である。本研究では、F-アクチンとミオシンの水和水ダイナミクスを中性子準弾性散乱を用いて解析した。J-PARCのBL14 AMATERASを用いてF-アクチン溶液とミオシン溶液(共に、H O又はD
O又はD Oバッファーの両方を調製)準弾性散乱測定を行った。水和水由来のスペクトルを抽出し、ローレンツ関数によるフィッティングによって、水分子の並進拡散係数及び滞留時間を算出した。現段階の解析では、ミオシンの並進拡散係数はバルク水よりも小さく、同時に滞留時間はバルク水よりも長いことが分かった。このことは、ミオシン水和水の運動性がバルク水よりも抑制されているという一般的なタンパク質水和水の特徴を示している。一方、F-アクチン水和水の並進拡散係数と滞留時間はバルク水に近く、F-アクチン水和水の運動性が通常のタンパク質水和水とは異なる可能性を示唆している。
Oバッファーの両方を調製)準弾性散乱測定を行った。水和水由来のスペクトルを抽出し、ローレンツ関数によるフィッティングによって、水分子の並進拡散係数及び滞留時間を算出した。現段階の解析では、ミオシンの並進拡散係数はバルク水よりも小さく、同時に滞留時間はバルク水よりも長いことが分かった。このことは、ミオシン水和水の運動性がバルク水よりも抑制されているという一般的なタンパク質水和水の特徴を示している。一方、F-アクチン水和水の並進拡散係数と滞留時間はバルク水に近く、F-アクチン水和水の運動性が通常のタンパク質水和水とは異なる可能性を示唆している。
In this study, we investigated the dynamics of the hydration water around F-actin and myosin S1, which are proteins interacting with each other to produce force in muscle contraction, by quasielastic neutron scattering (QENS). The QENS measurements were conducted on the solution samples of F-actin (150 mg/ml) and S1 (80 mg/ml) in H O and D
O and D O using the cold-neutron disk-chopper spectrometer AMATERAS in MLF/J-PARC, Japan. The spectra of hydration water were obtained by subtracting those of proteins and those of bulk water from the measured spectra of H
O using the cold-neutron disk-chopper spectrometer AMATERAS in MLF/J-PARC, Japan. The spectra of hydration water were obtained by subtracting those of proteins and those of bulk water from the measured spectra of H O samples (the spectra of proteins were obtained by subtracting those of D
O samples (the spectra of proteins were obtained by subtracting those of D O buffer from those of D
O buffer from those of D O samples, while the spectra of H
O samples, while the spectra of H O buffer was used as those of bulk water) with appropriate scaling factors. The translational diffusion coefficients(D
O buffer was used as those of bulk water) with appropriate scaling factors. The translational diffusion coefficients(D ) and the residence time (
) and the residence time (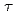
 ) were evaluated from the dependence of the half-widths at half-maximum of the Lorentzian functions fit to the spectra on the momentum transfer. In the current analysis, it was found that the D
) were evaluated from the dependence of the half-widths at half-maximum of the Lorentzian functions fit to the spectra on the momentum transfer. In the current analysis, it was found that the D value of the hydration water around S1was smaller than that of bulk water while the
value of the hydration water around S1was smaller than that of bulk water while the 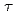
 value was larger than that of bulk water, suggesting that S1 has typical hydration water, the mobility of which is less than that of bulkwater. On the other hand, both the D
value was larger than that of bulk water, suggesting that S1 has typical hydration water, the mobility of which is less than that of bulkwater. On the other hand, both the D and the
and the 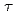
 values of the hydration water around F-actin were closer to those of bulk water, suggesting that the hydration water around F-actin has higher mobility than that around other proteins including S1. The results of a more detailed analysis will be given in the presentation.
values of the hydration water around F-actin were closer to those of bulk water, suggesting that the hydration water around F-actin has higher mobility than that around other proteins including S1. The results of a more detailed analysis will be given in the presentation.