Development of 2-[ At]astato-
At]astato- -methyl-L-phenylalanine (2-AAMP) as a novel radiopharmaceutical for internal radiotherapy
-methyl-L-phenylalanine (2-AAMP) as a novel radiopharmaceutical for internal radiotherapy
内用放射線療法のための新規放射性薬剤2-[ At]astato-
At]astato- -methyl-L-phenylalanine (2-AAMP)の開発
-methyl-L-phenylalanine (2-AAMP)の開発
大島 康宏; 鈴木 博元*; 花岡 宏史*; 渡辺 茂樹; 渡辺 智; 渡邉 直行*; 対馬 義人*; 遠藤 啓吾*; 荒野 泰*; 石岡 典子
Ohshima, Yasuhiro; Suzuki, Hiroyuki*; Hanaoka, Hirofumi*; Watanabe, Shigeki; Watanabe, Satoshi; Watanabe, Naoyuki*; Tsushima, Yoshito*; Endo, Keigo*; Arano, Yasushi*; Ishioka, Noriko
In this study, we newly synthesized 2-[ At]astato-
At]astato- -methyl-Lphenylalanine (2-AAMP) and investigated its possibility as a novel radiopharmaceutical for the internal radiotherapy.
-methyl-Lphenylalanine (2-AAMP) and investigated its possibility as a novel radiopharmaceutical for the internal radiotherapy.  At was produced via the
At was produced via the 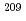 Bi(
Bi( , 2n)
, 2n) At reaction and separated from the Bi target by a dry-distillation technique. For 2-AAMP synthesis, 2-trimethylstannyl-
At reaction and separated from the Bi target by a dry-distillation technique. For 2-AAMP synthesis, 2-trimethylstannyl- -trifluoroacetyl-
-trifluoroacetyl- -methyl-L-phenylalanine methyl ester was synthesized as a precursor and radioastatinated in the presence of
-methyl-L-phenylalanine methyl ester was synthesized as a precursor and radioastatinated in the presence of  -chlorosuccinimide. The following deprotection reaction gave 2-AAMP in 18% yield. 2-AAMP was rapidly taken up into SKOV3 cells expressing L-type amino acid transporter 1 (LAT1) which is highly and specifically expressed in tumor cells. The uptake of 2-AAMP was significantly inhibited by not only the treatment with L-amino acids which are the substrates of LAT1, but also 2-amino-bicyclo[2,2,1]heptane-2-carboxylic acid, an inhibitor of LAT, indicating that 2-AAMP could be taken up through LAT1. Colony formation of SKOV3 was decreased to approx. 46% of control by the treatment with 2-AAMP for 22 h at 25 kBq/ml (initial concentration of radioactivity). These results suggest that 2-AAMP might be selectively accumulated in tumor and suppress growth of cancer. At present, stability, biodistribution and anti-tumor activity of 2-AAMP are under investigation.
-chlorosuccinimide. The following deprotection reaction gave 2-AAMP in 18% yield. 2-AAMP was rapidly taken up into SKOV3 cells expressing L-type amino acid transporter 1 (LAT1) which is highly and specifically expressed in tumor cells. The uptake of 2-AAMP was significantly inhibited by not only the treatment with L-amino acids which are the substrates of LAT1, but also 2-amino-bicyclo[2,2,1]heptane-2-carboxylic acid, an inhibitor of LAT, indicating that 2-AAMP could be taken up through LAT1. Colony formation of SKOV3 was decreased to approx. 46% of control by the treatment with 2-AAMP for 22 h at 25 kBq/ml (initial concentration of radioactivity). These results suggest that 2-AAMP might be selectively accumulated in tumor and suppress growth of cancer. At present, stability, biodistribution and anti-tumor activity of 2-AAMP are under investigation.