Synergistic solvent extraction of lanthanide ions with mixtures of D2EHPA and MIDPA in phosphonium-based ionic liquids
D2EHPAとMIDPAを用いる希土類元素のイオン液体中への協同抽出
松宮 正彦*; 野水 大輝*; 土田 裕介*; 佐々木 祐二 

Matsumiya, Masahiko*; Nomizu, Daiki*; Tsuchida, Yusuke*; Sasaki, Yuji
D2EHPA(A)とMIDPA(B)を用いる希土類元素のイオン液体中へ協同抽出が検討された。D2EHPAまたはMIDPAを単独で抽出に用いる際には、その抽出種は[LnA HA]か[LnB
HA]か[LnB HB]であった。錯形成定数から、協同抽出錯体である[TbHA
HB]であった。錯形成定数から、協同抽出錯体である[TbHA B
B ]や[DyHA
]や[DyHA B
B ]は単独抽出剤による[LnA
]は単独抽出剤による[LnA HA],[LnB
HA],[LnB HB]等の錯体種より安定であることが確認された。分離係数を考慮すると、協同効果を用いてDyをPrやNdからの分離が可能であることを確認した。
HB]等の錯体種より安定であることが確認された。分離係数を考慮すると、協同効果を用いてDyをPrやNdからの分離が可能であることを確認した。
The synergistic solvent extraction of lanthanide(III) with mixtures of di-(2-ethylhexyl)phosphoric acid (D2EHPA, A) and monoisodecyl phosphoric acid (MIDPA, B) in phosphonium-based ionic liquid was investigated. In the case of D2EHPA or MIDPA single extractant system, Ln(III) (Ln = Pr and Nd) was extracted as [LnA HA] or [LnB
HA] or [LnB HB], respectively, the extracted species of Tb(III) or Dy(III) were determined by slope analysis. According to the equilibrium constants (
HB], respectively, the extracted species of Tb(III) or Dy(III) were determined by slope analysis. According to the equilibrium constants (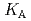 ,
, 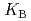 and
and 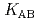 ) and the formation constants (
) and the formation constants (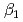 ,
, 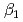 and
and 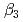 ), it was found that the extracted complex [TbHA
), it was found that the extracted complex [TbHA B
B or [DyHA
or [DyHA B
B ] was more stable than [LnA
] was more stable than [LnA HA] or [LnB
HA] or [LnB HB]. The synergistic extraction effects were investigated to study the possibility of separating Dy(III) from Pr(III) and Nd(III) according to their separation factors.
HB]. The synergistic extraction effects were investigated to study the possibility of separating Dy(III) from Pr(III) and Nd(III) according to their separation factors.