Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Rizaal, M.; 中島 邦久; 鈴木 恵理子; 三輪 周平
Annals of Nuclear Energy, 218, p.111433_1 - 111433_10, 2025/08
被引用回数:0 パーセンタイル:0.00(Nuclear Science & Technology)The release of iodine in a case of severe nuclear accident is directly linked to short-term radiological consequences. This concern raises issues in understanding the chemical forms of the transported iodine to devise proper accident management measures/strategies. In contributing to such efforts, this work presents experimental and theoretical approaches to defining the impact of molybdenum as a semi-volatile fission product toward iodine speciation in the gas phase. Given humid atmospheric conditions with different oxygen potentials, the interactions were revealed through the reaction products consisting of both gas and aerosols upon their transport and condensation in the temperature range of 1150-450 K. Through thermodynamic equilibrium calculations where new thermodynamic data of cesium molybdates have been incorporated, the experimental observation was reproduced, hence general interaction mechanism was proposed in this work.
 and Fe-Zr melt
and Fe-Zr melt中島 邦久; 高野 公秀
Journal of Nuclear Science and Technology, 62(1), p.78 - 85, 2025/01
被引用回数:1 パーセンタイル:51.66(Nuclear Science & Technology)1Fでは、シビアアクシデント解析コードを用いた解析や汚染水からの逆解析により相当量のCsが炉内にまだ残っていると推定されている。そのため、炉心領域で想定されるCs蒸気とUO あるいはFe-Zr融体との化学的な相互作用の有無を調べた。その結果、Cs
あるいはFe-Zr融体との化学的な相互作用の有無を調べた。その結果、Cs UO
UO やCs
やCs ZrO
ZrO の生成が確認され、燃料から放出されたCsがUO
の生成が確認され、燃料から放出されたCsがUO 燃料やFe-Zr融体と化学的な相互作用により付着する可能性があることが分かった。
燃料やFe-Zr融体と化学的な相互作用により付着する可能性があることが分かった。
 -CsOH
-CsOHRizaal, M.; Luu, V. N.; 中島 邦久; 三輪 周平
Proceedings of International Topical Workshop on Fukushima Decommissioning Research 2024 (FDR2024) (Internet), 4 Pages, 2024/10
Thermochemistry prevailing between gaseous CsOH and concrete main chemical phase CaCO at temperatures up to 570
at temperatures up to 570 C was investigated with various scenarios using the thermogravimetric method. The aim was to elucidate the decreasing behavior of cesium (Cs) trapping on CaCO
C was investigated with various scenarios using the thermogravimetric method. The aim was to elucidate the decreasing behavior of cesium (Cs) trapping on CaCO observed in the transpiration method. A quasi-two-compartment platinum crucible was developed to realize co-measurements of both CsOH and CaCO
observed in the transpiration method. A quasi-two-compartment platinum crucible was developed to realize co-measurements of both CsOH and CaCO during thermal treatment. Post-test X-ray diffraction was conducted to identify the chemical compound formed on the CaCO
during thermal treatment. Post-test X-ray diffraction was conducted to identify the chemical compound formed on the CaCO precursor. The early presence (timely sensitivity) of CsOH near the heated surface of CaCO
precursor. The early presence (timely sensitivity) of CsOH near the heated surface of CaCO was found to play a key role in the trapping (in the form of Cs
was found to play a key role in the trapping (in the form of Cs CO
CO ). Such a factor is crucial because, otherwise, the Ca(OH)
). Such a factor is crucial because, otherwise, the Ca(OH) would predominate the surface upon CaCO
would predominate the surface upon CaCO decomposition where leading to no reaction with CsOH.
decomposition where leading to no reaction with CsOH.
Rizaal, M.; 中島 邦久
Chemosphere, 363, p.142870_1 - 142870_9, 2024/09
Retention or trapping of cesium, one of the radiologically important fission products, in the nuclear reactor becomes a great concern as the occurrence may affect radioactivity in the long term or its environmental fate. Herein the chemical compound of cesium that had been largely trapped on the nuclear reactor structural material of (calcium silicate) thermal insulator in a simulated nuclear accident condition was investigated. A combined pre- and post-water dissolution analysis through infrared (IR) spectroscopy and optical emission spectroscopy (OES) was explored to resolve the characterization difficulty encountered in conventional X-ray diffraction analysis reported in the previous works. This method allowed us to identify for the first time the related large amount of water-soluble cesium in the calcium silicate material after a high-temperature chemical reaction as cesium metasilicate (Cs SiO
SiO ). It was evidenced by similar vibrational characteristics of the material to that in the synthesized Cs
). It was evidenced by similar vibrational characteristics of the material to that in the synthesized Cs SiO
SiO as well as the dissolved Cs and Si in the leaching water having a molar ratio of 2.16
as well as the dissolved Cs and Si in the leaching water having a molar ratio of 2.16 0.33. The corresponding 79-98% of the retained cesium in calcium silicate materials in the case study of 700 and 800
0.33. The corresponding 79-98% of the retained cesium in calcium silicate materials in the case study of 700 and 800 C reactions was of this compound, emphasizing its significance once formed. Thermodynamic considerations further corroborated the higher stability of Cs
C reactions was of this compound, emphasizing its significance once formed. Thermodynamic considerations further corroborated the higher stability of Cs SiO
SiO in the cesium-calcium silicate reaction than other cesium silicates such as Cs
in the cesium-calcium silicate reaction than other cesium silicates such as Cs Si
Si O
O , Cs
, Cs Si
Si O
O , or Cs
, or Cs Si
Si O
O . This clearly poses a high environmental risk due to the volatility of cesium metasilicate as it may spread out further through the water leak path from a damaged nuclear reactor.
. This clearly poses a high environmental risk due to the volatility of cesium metasilicate as it may spread out further through the water leak path from a damaged nuclear reactor.
 at temperature range 170 - 290
at temperature range 170 - 290 C
CLuu, V. N.; 中島 邦久
Nuclear Engineering and Design, 426, p.113402_1 - 113402_7, 2024/09
被引用回数:0 パーセンタイル:0.00(Nuclear Science & Technology)A field assessment at the Fukushima-Daiichi Nuclear Power Station revealed high radioactivity on the concrete shield plugs, which is estimated above 20 PBq for Cs-137 at units 2 and 3. This leads to significant interest in the retention of Cs on concrete during severe accidents (SA). However, the interaction of CsOH, as one of the main Cs forms released in SA, with concrete surfaces at elevated temperatures remains poorly researched. In this study, we have experimentally investigated the deposition behavior of CsOH on CaCO , which is the primary phase existing on the surface of concrete, under humid atmosphere. As a result, the chemical reaction enhanced deposition rate (N), and increased linearly with CsOH concentration (C
, which is the primary phase existing on the surface of concrete, under humid atmosphere. As a result, the chemical reaction enhanced deposition rate (N), and increased linearly with CsOH concentration (C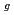 ), as following expression: N(
), as following expression: N( g/cm
g/cm
 s) = v
s) = v C
C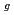 , where v
, where v is temperature-dependent deposition velocity as given by ln v
is temperature-dependent deposition velocity as given by ln v (cm/s) = -3785.8/T + 3.766, for T in the range of 170 and 290
(cm/s) = -3785.8/T + 3.766, for T in the range of 170 and 290  C. This empirical model can be integrated into severe accident codes to quantify the chemical trapping of cesium on concrete surfaces during ex-vessel release. Moreover, it can contribute to understanding the reasons behind the high dose rate on concrete shield plugs at the Fukushima Daiichi Nuclear power stations and aid in developing effective decommissioning practices for concrete structures.
C. This empirical model can be integrated into severe accident codes to quantify the chemical trapping of cesium on concrete surfaces during ex-vessel release. Moreover, it can contribute to understanding the reasons behind the high dose rate on concrete shield plugs at the Fukushima Daiichi Nuclear power stations and aid in developing effective decommissioning practices for concrete structures.
 reaction system
reaction systemRizaal, M.; 中島 邦久; 鈴木 恵理子; 三輪 周平
Proceedings of 11th European Review Meeting on Severe Accident Research Conference (ERMSAR 2024) (Internet), 11 Pages, 2024/05
The pseudo-binary CsI-MoO reaction system during transportation in the reactor under severe accident conditions has been investigated by using the JAEA-TeRRa semi-integral test facility. An oxygen gas with approximately 10 mbar of partial pressure was given to argon-steam upstream gas flow during the reaction to study its effect on downstream chemistry between CsI and MoO
reaction system during transportation in the reactor under severe accident conditions has been investigated by using the JAEA-TeRRa semi-integral test facility. An oxygen gas with approximately 10 mbar of partial pressure was given to argon-steam upstream gas flow during the reaction to study its effect on downstream chemistry between CsI and MoO (Mo/Cs molar ratio of 2.13). The product of CsI-MoO
(Mo/Cs molar ratio of 2.13). The product of CsI-MoO reactions both gas and aerosols were analyzed upon their condensation on type-304L stainless steel sampling coupons at respective temperatures (T= 1150-450 K). Post-test analyses revealed that at a low oxygen potential of -192 kJ/mol (i.e. reference case with argon-steam only), most of the CsI reached downstream (T
reactions both gas and aerosols were analyzed upon their condensation on type-304L stainless steel sampling coupons at respective temperatures (T= 1150-450 K). Post-test analyses revealed that at a low oxygen potential of -192 kJ/mol (i.e. reference case with argon-steam only), most of the CsI reached downstream (T  400 K) without any reaction with MoO
400 K) without any reaction with MoO . On the other hand, when oxygen potential was slightly increased to about -144 kJ/mol, the CsI vapor could react with MoO
. On the other hand, when oxygen potential was slightly increased to about -144 kJ/mol, the CsI vapor could react with MoO to form cesium polymolybdates (Cs
to form cesium polymolybdates (Cs Mo
Mo O
O and Cs
and Cs Mo
Mo O
O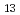 ) and gaseous iodine which predominated the aerosol and gas that reached the downstream region. Particle size at this location was found to be less than 2.7
) and gaseous iodine which predominated the aerosol and gas that reached the downstream region. Particle size at this location was found to be less than 2.7  m in contrast to the former case having an irregularly large size. The gaseous iodine in the latter case, based on the thermodynamic analyses, was estimated to be hypoiodous acid (HIO) or molecular iodine (I
m in contrast to the former case having an irregularly large size. The gaseous iodine in the latter case, based on the thermodynamic analyses, was estimated to be hypoiodous acid (HIO) or molecular iodine (I ). The results in this study indicated that speciation of both Cs and I with the Mo chemistry during a severe accident could be variedly formed depending on the prevailing oxygen potential.
). The results in this study indicated that speciation of both Cs and I with the Mo chemistry during a severe accident could be variedly formed depending on the prevailing oxygen potential.
Li, N.*; Sun, Y.*; 中島 邦久; 黒崎 健*
Journal of Nuclear Science and Technology, 61(3), p.343 - 353, 2024/03
被引用回数:1 パーセンタイル:23.64(Nuclear Science & Technology)福島原子力発電所(1F)事故では、表面積の大きなステンレス鋼(SS304)製の気水分離器や蒸気乾燥器にセシウムが大量に残っている可能性がある。そして、1F廃止措置においてこのようなCsは、放射性粉塵を生成する可能性があるため、安全上問題になることが予想される。しかし、水酸化セシウム(CsOH)の化学吸着により生成した酸化被膜の付着強度については、まだ、明らかになっていない。本研究では、CsOHによる化学吸着がどの程度酸化被膜の付着強度に影響するかスクラッチ試験機を用いて調査した。その結果、CsOHの化学吸着により酸化被膜の付着強度は低下したが、剥離させることはできなかった。
 C
CLuu, V. N.; 中島 邦久
Mechanical Engineering Journal (Internet), 11(2), p.23-00446_1 - 23-00446_11, 2024/01
Cesium distribution is crucial for decommissioning Fukushima Daiichi Nuclear Power Station (1F). Several experimental studies confirmed Cs retention on stainless steels by performing chemical reactions at high temperatures (typically above 800 C)), but the Cs retention on concrete, used in large quantities in light water reactors, is not fully understood. This study demonstrated that Cs might have been deposited and retained on the concrete structures where the temperature was not so high during the 1F accident. Results showed that the CsOH/concrete interaction at around 200
C)), but the Cs retention on concrete, used in large quantities in light water reactors, is not fully understood. This study demonstrated that Cs might have been deposited and retained on the concrete structures where the temperature was not so high during the 1F accident. Results showed that the CsOH/concrete interaction at around 200 C occurred in water-insoluble Cs-(Al,Fe)-Si-O deposits and water-soluble phases, i.e., cesium carbonate hydrate and possibly cesium silicate if Al and Fe are not present. CsOH might be trapped on concrete by chemical reaction with CaCO
C occurred in water-insoluble Cs-(Al,Fe)-Si-O deposits and water-soluble phases, i.e., cesium carbonate hydrate and possibly cesium silicate if Al and Fe are not present. CsOH might be trapped on concrete by chemical reaction with CaCO to form Cs
to form Cs CO
CO hydrate, and with aluminosilicate and SiO
hydrate, and with aluminosilicate and SiO (quartz) to form Cs-Al-Si-O and Cs-Si-O deposits, respectively. This output could help elucidate the trapping mechanism that caused extremely high radioactivity on concrete shield plugs at 1F and develop an effective decommissioning practice for concrete structures.
(quartz) to form Cs-Al-Si-O and Cs-Si-O deposits, respectively. This output could help elucidate the trapping mechanism that caused extremely high radioactivity on concrete shield plugs at 1F and develop an effective decommissioning practice for concrete structures.
 C
CLuu, V. N.; 中島 邦久
Proceedings of 30th International Conference on Nuclear Engineering (ICONE30) (Internet), 9 Pages, 2023/05
Information of Cs distribution is important for decommissioning of the Fukushima Daiichi Nuclear Power Station (1F). Several experimental studies confirmed the Cs retention on stainless steels by chemical reaction at very high temperatures (commonly above 800 C), but the Cs retention on non-metallic materials, such as concrete and thermal insulators, was not fully understood though they are used with large quantity in light water reactors. This study demonstrated that Cs might be deposited and retained on the concrete structure where the temperature was not so high during the 1F accident. It was revealed that the CsOH/concrete interaction at around 200
C), but the Cs retention on non-metallic materials, such as concrete and thermal insulators, was not fully understood though they are used with large quantity in light water reactors. This study demonstrated that Cs might be deposited and retained on the concrete structure where the temperature was not so high during the 1F accident. It was revealed that the CsOH/concrete interaction at around 200 C resulted in the formation of water-insoluble Cs-(Al,Fe)-Si-O deposits and water-soluble phases, i.e., cesium carbonate hydrate and possibly cesium silicate, if Al and Fe are not present. CsOH might be trapped on concrete by chemical reaction with CaCO
C resulted in the formation of water-insoluble Cs-(Al,Fe)-Si-O deposits and water-soluble phases, i.e., cesium carbonate hydrate and possibly cesium silicate, if Al and Fe are not present. CsOH might be trapped on concrete by chemical reaction with CaCO to form Cs
to form Cs CO
CO hydrate, and with aluminosilicate and SiO
hydrate, and with aluminosilicate and SiO (quartz) to form Cs-Al-Si-O and Cs-Si-O deposits, respectively. This output will be useful for elucidating the trapping mechanism that caused an extremely high radioactivity on concrete shield plugs at 1F, and for developing an effective decommissioning practice for concrete structure.
(quartz) to form Cs-Al-Si-O and Cs-Si-O deposits, respectively. This output will be useful for elucidating the trapping mechanism that caused an extremely high radioactivity on concrete shield plugs at 1F, and for developing an effective decommissioning practice for concrete structure.
Mohamad, A. B.; 中島 邦久; 三輪 周平; 逢坂 正彦
Journal of Nuclear Science and Technology, 60(3), p.215 - 222, 2023/03
被引用回数:0 パーセンタイル:0.00(Nuclear Science & Technology)The distribution of Sr in the reactor would be influenced by a chemical reaction of Sr vapor species with a structural material of internal reactor and fuel cladding materials; stainless steel (SS) or Zircaloy (Zry) cladding during 1F-NPS accident. The chemical interaction between Sr-Zry and Sr-SS has been described. The reaction tests have been performed to investigate the chemical interaction behavior under possible severe accident conditions. The tests have been conducted up to 1523 K under steam atmosphere. It was confirmed that Sr-Zr-O and Sr-Si-O compounds were formed through 2 kinds chemical interactions; gas-solid reaction and liquid-solid reaction. The gas and liquid species of Sr in a good contact with the solid Zry and SS to form Sr-Zr-O and Sr-Si-O compounds, respectively. Sr was deposited onto the Zry and SS surfaces and lead to the formation of reaction product. Thus, this study highlights the possibility that Sr was deposited and retained in the core structure where the temperature was elevated during the accident in the 1F-NPS.
Luu, V. N.; 中島 邦久
Journal of Nuclear Science and Technology, 60(2), p.153 - 164, 2023/02
被引用回数:6 パーセンタイル:67.31(Nuclear Science & Technology)Recently, extremely high dose rates were detected in the three-layer concrete plugs of Units 2 and 3 at the Fukushima Daiichi Nuclear Power Plant. The high dose rates suggest that there are some trapping effects of radioactive materials on shield plugs when gas species and aerosols (e.g., CsOH, CsI) are released from reactor through the plug layers. To determine the trapping mechanism, concrete and commonly used aggregate and minerals are pulverized and mixed with CsOH, followed by heating at different temperatures to clarify the chemical interaction. The results showed that interactions of CsOH and CaCO in concrete occurred even at room temperature to form Cs
in concrete occurred even at room temperature to form Cs CO
CO (H
(H O)
O) . The interaction with aggregates occurred above 100
. The interaction with aggregates occurred above 100 C and resulted in the formation of CsAlSiO
C and resulted in the formation of CsAlSiO . Additionally, amorphous and crystalline SiO
. Additionally, amorphous and crystalline SiO interacted with CsOH, forming a glass-like product above 200
interacted with CsOH, forming a glass-like product above 200 C. These results suggest that formation of Cs
C. These results suggest that formation of Cs CO
CO (H
(H O)
O) would be one of the main trapping mechanism at shield plugs because CaCO
would be one of the main trapping mechanism at shield plugs because CaCO is commonly formed on concrete surface and reacts with CsOH at room temperature.
is commonly formed on concrete surface and reacts with CsOH at room temperature.
三輪 周平; 唐澤 英年; 中島 邦久; 木野 千晶*; 鈴木 恵理子; 井元 純平
JAEA-Data/Code 2021-022, 32 Pages, 2023/01
東京電力福島第一原子力発電所の原子炉内におけるセシウム分布のより正確な予測に向けて、核分裂生成物の化学挙動データベースECUMEに格納されているステンレス鋼へのセシウム化学吸着モデルをシビアアクシデント解析コードSAMPSONに組み込んだ。改良モデルを組み込んだSAMPSONにより、当該モデルを構築した実験の結果を再現し、コードに誤りが無いことを確認した。また、SAMPSONに組み込まれた改良モデルのセシウム化学着挙動解析への有効性を確認するため、温度勾配管を有する装置を用いた実験の解析を実施した。改良モデルを組み込んだSAMPSONにより、実験の結果を再現し、SAMPSONにおけるノードジャンクションの設定方法、エアロゾル生成モデル、CsOH蒸気の飽和蒸気圧等の計算方法等の解析方法、そして改良モデルがセシウム化学吸着挙動解析に適用可能であることを確認した。また、セシウムがシビアアクシデント後に水相を介して移行したことから、原子炉内におけるセシウム分布を予測するためには、セシウム沈着物の水への溶解性の評価が前提となる。このため、ステンレス鋼へのセシウム化学吸着生成物の水への溶解性を調べた。ステンレス鋼304へのセシウム化学吸着生成物は、873Kから973Kで水溶性の高いCsFeO 、973Kから1273Kで水溶性の低いCsFeSiO
、973Kから1273Kで水溶性の低いCsFeSiO 、1073Kから1273Kで水溶性の低いCs
、1073Kから1273Kで水溶性の低いCs Si
Si O
O であることが分かった。これらの結果から、セシウム化学吸着量に影響を与える原子炉内温度やCsOH蒸気種濃度のようなシビアアクシデント解析条件に応じて、セシウム化学吸着生成物の水への溶解性を予測できる可能性を得た。
であることが分かった。これらの結果から、セシウム化学吸着量に影響を与える原子炉内温度やCsOH蒸気種濃度のようなシビアアクシデント解析条件に応じて、セシウム化学吸着生成物の水への溶解性を予測できる可能性を得た。
Rizaal, M.; 中島 邦久; 斉藤 拓巳*; 逢坂 正彦; 岡本 孝司*
ACS Omega (Internet), 7(33), p.29326 - 29336, 2022/08
被引用回数:5 パーセンタイル:35.08(Chemistry, Multidisciplinary)Here we report an investigation of the gas-solid reaction between cesium hydroxide (CsOH) and siliceous (calcium silicate) thermal insulation at high temperature, which was postulated as the origin for the formation mechanism of cesium-bearing material emitted from the Fukushima Daiichi Nuclear Power Plant. A developed reaction furnace consisting of two heating compartments was used to study the reaction at temperatures of 873, 973, and 1073 K. Under the influence of hydrogen-steam atmospheric conditions (H /H
/H O = 0.2), the reaction between cesium hydroxide vapor and solid thermal insulation was confirmed to occur at temperatures of 973 and 1073 K with the formation of dicalcium silicate (Ca
O = 0.2), the reaction between cesium hydroxide vapor and solid thermal insulation was confirmed to occur at temperatures of 973 and 1073 K with the formation of dicalcium silicate (Ca SiO
SiO ) and cesium aluminum silicate (CsAlSiO
) and cesium aluminum silicate (CsAlSiO ). Water-dissolution analyses of the reaction products have demonstrated their stability, in particular, the CsAlSiO
). Water-dissolution analyses of the reaction products have demonstrated their stability, in particular, the CsAlSiO . Constituents similarity of the field-observed cesium-bearing materials near the Fukushima Daiichi Nuclear Power Plants with CsAlSiO
. Constituents similarity of the field-observed cesium-bearing materials near the Fukushima Daiichi Nuclear Power Plants with CsAlSiO suggests for the first time that gaseous reaction between CsOH with calcium silicate thermal insulation could be one of the original formation mechanisms of the cesium-bearing materials.
suggests for the first time that gaseous reaction between CsOH with calcium silicate thermal insulation could be one of the original formation mechanisms of the cesium-bearing materials.
Liu, J.; 中島 邦久; 三輪 周平; 白数 訓子; 逢坂 正彦
Journal of Nuclear Science and Technology, 59(4), p.484 - 490, 2022/04
被引用回数:0 パーセンタイル:0.00(Nuclear Science & Technology)A new method named TG calibration curve method has been proposed to evaluate the Ru activity in the Ru-containing alloys by using the kinetic relationship between oxidative evaporation rate of Ru atom and overall vapor pressures of various RuOx gases in the experimental system constructed by the authors. The experimental results show that the oxidative evaporation rate of Ru atom from experimental powders is approximately proportional to the overall vapor pressures over powders. By using their specific quantitative relationship, we determined the Ru activity in Ru Pd
Pd alloy in the temperature range from 1523 to 1673 K. The obtained values of Ru activity agree well with those theoretically calculation, and indicated the reliability of this new method.
alloy in the temperature range from 1523 to 1673 K. The obtained values of Ru activity agree well with those theoretically calculation, and indicated the reliability of this new method.
鈴木 知史; 中島 邦久; 逢坂 正彦
Journal of Nuclear Science and Technology, 59(3), p.345 - 356, 2022/03
被引用回数:3 パーセンタイル:30.71(Nuclear Science & Technology)福島第一原子力発電所事故などの過酷事故(SA)では、原子炉の構造材料の表面に核分裂生成物(FP)が沈着する可能性がある。FPの中でも特にセシウム(Cs)は重要であり、SA発生時には原子炉内の構造材のステンレス鋼(SS)の表面にCsケイ酸塩等の種々のCs化合物が形成される。これらのCs化合物の相安定性を評価するために、Cs-Si-O化合物のエネルギーの計算を実施した。その結果、Cs Si
Si O
O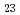 がCs-Si-O化合物の中で最も安定していることが分かった。さらに、Cs-Si-Fe-O化合物のエネルギーの計算を実施した。計算結果より、Cs-Si-Fe-O化合物はCs-Si-O化合物よりも安定しており、CsSi
がCs-Si-O化合物の中で最も安定していることが分かった。さらに、Cs-Si-Fe-O化合物のエネルギーの計算を実施した。計算結果より、Cs-Si-Fe-O化合物はCs-Si-O化合物よりも安定しており、CsSi FeO
FeO がCs-Si-O化合物およびCs-Si-Fe-O化合物の中で最も安定していることが分かった。今回の計算と過去の実験の結果によって、Cs-Si-Fe-O化合物はCs化学吸着によってSS表面に安定して形成できることが示された。
がCs-Si-O化合物およびCs-Si-Fe-O化合物の中で最も安定していることが分かった。今回の計算と過去の実験の結果によって、Cs-Si-Fe-O化合物はCs化学吸着によってSS表面に安定して形成できることが示された。
逢坂 正彦; Gou llo, M.*; 中島 邦久
llo, M.*; 中島 邦久
Journal of Nuclear Science and Technology, 59(3), p.292 - 305, 2022/03
被引用回数:7 パーセンタイル:61.36(Nuclear Science & Technology)事故時及び長期間の2つの期間のソースタームにおけるセシウム化学について、福島第一原子力発電所(1F)事故後に行われたFP化学研究のレビューを行った。事故時についてはCsのMo, B, Siとの化学反応について、また1F固有の水相を介した長期についてはCsのコンクリートへの浸透及び燃料デブリの浸出挙動について、関連する熱力学データ整備状況とともに調べた。これらのCs化学挙動は近い将来取出し予定の燃料デブリ等1Fサンプルの分析及び評価を通して検証されるべきである。
井元 純平; 中島 邦久; 逢坂 正彦
日本原子力学会和文論文誌, 20(4), p.179 - 187, 2021/12
福島第一原子力発電所の炉内や原子炉建屋内のCsの一部は、CsIやCs MoO
MoO といった化学形で沈着すると考えられているが、Cs化合物の多くは水溶性であるため長期的には水相を介した移行が起きると推察される。このような移行挙動評価のための基礎データとして、水への溶解度に関する知見が必要である。そのため、本研究では20
といった化学形で沈着すると考えられているが、Cs化合物の多くは水溶性であるため長期的には水相を介した移行が起きると推察される。このような移行挙動評価のための基礎データとして、水への溶解度に関する知見が必要である。そのため、本研究では20 C及び25
C及び25 CにおけるCsIとCs
CにおけるCsIとCs MoO
MoO の水への溶解度について調べることを目的とした。その結果、熱力学的データとPitzerモデルから計算された25
の水への溶解度について調べることを目的とした。その結果、熱力学的データとPitzerモデルから計算された25 CでのCsIの溶解度は、文献値とよく一致していることが示され、室温付近のCsIの文献値は信頼性が高いことが分かった。OECDテストガイドライン105フラスコ法(テストガイドライン)を使用した20
CでのCsIの溶解度は、文献値とよく一致していることが示され、室温付近のCsIの文献値は信頼性が高いことが分かった。OECDテストガイドライン105フラスコ法(テストガイドライン)を使用した20 CでのCsIの実験値も、文献値とよく一致していた。テストガイドラインを使用して測定した20
CでのCsIの実験値も、文献値とよく一致していた。テストガイドラインを使用して測定した20 CのCs
CのCs MoO
MoO の溶解度は、256.8
の溶解度は、256.8 6.2(g/100g H
6.2(g/100g H O)となった。このCs
O)となった。このCs MoO
MoO の溶解度は、他のアルカリモリブデン酸塩の溶解度と同程度であることがわかり、文献値よりも信頼性が高いと考えられる。
の溶解度は、他のアルカリモリブデン酸塩の溶解度と同程度であることがわかり、文献値よりも信頼性が高いと考えられる。
三輪 周平; 宮原 直哉*; 中島 邦久; 井元 純平; 鈴木 恵理子
日本原子力学会誌ATOMO , 63(12), p.825 - 829, 2021/12
, 63(12), p.825 - 829, 2021/12
沸騰水型原子炉のシビアアクシデント時において、制御材ホウ素は、被ばく低減等の観点で重要なセシウム等の化学挙動や移行挙動に大きな影響を与えることが示唆されており、環境放出量や炉内分布の予測に内在する大きな不確かさの要因であった。そこで、シビアアクシデント解析で考慮すべき重要な化学挙動を解明するため、炉内移行時の化学挙動評価を可能とする装置を開発して、セシウムの化学挙動を評価した。その結果を基に、シビアアクシデント解析コードで化学挙動を評価できるように化学反応解析の基盤となるデータセットやモデルから構成されるデータベースECUMEを整備した。
Liu, J.; 三輪 周平; 中島 邦久; 逢坂 正彦
Nuclear Materials and Energy (Internet), 26, p.100916_1 - 100916_6, 2021/03
被引用回数:3 パーセンタイル:30.71(Nuclear Science & Technology)A new synthesis method of simulated fuel containing highly volatile compound of cesium iodide (CsI) was established based on a gas-tight sintering technique. This method can enhance the grain growth of the SIMFUEL pellets by increasing sintering temperature and time. The CsI content in the simulated fuel did not significantly decrease even for 10 hours sintering time and its final value can be quantitatively controlled by adjusting its vaporization amount. A slower heating rate after the melting of CsI can promote the dispersion of liquid CsI on the grain boundaries and give a very small CsI particle size.
三輪 周平; 中島 邦久; 鈴木 知史; Rizaal, M.; 鈴木 恵理子; 堀口 直樹; 逢坂 正彦
Proceedings of Joint International Conference on Supercomputing in Nuclear Applications + Monte Carlo 2020 (SNA + MC 2020), p.253 - 260, 2020/12
シビアアクシデント時のFP挙動評価における主な課題であるFP化学挙動評価と解析の空間分解能を改良するための基盤研究を行っている。FP化学挙動に関しては、SA解析コード改良に資する化学挙動データベースECUMEを開発しており、計算科学的なアプローチにより実験データの無いCs化合物の熱力学データを取得した。また、空間分解能に関しては、FPの詳細挙動解析が可能な3D-CFD解析ツールCHASERを開発している。ECUMEをCHASERに組み込むことでより正確なFP挙動解析が可能となる。