Crystallization and preliminary neutron diffraction studies of ADP-ribose pyrophosphatase-I from  HB8
HB8
好熱菌 HB8由来ADPリボースピロホスファターゼIの結晶化及び中性子回折研究
HB8由来ADPリボースピロホスファターゼIの結晶化及び中性子回折研究
岡崎 伸生; 安達 基泰; 玉田 太郎; 栗原 和男; 大賀 拓史*; 神谷 信夫*; 倉光 成紀*; 黒木 良太
Okazaki, Nobuo; Adachi, Motoyasu; Tamada, Taro; Kurihara, Kazuo; Oga, Takushi*; Kamiya, Nobuo*; Kuramitsu, Seiki*; Kuroki, Ryota
ADP-ribose pyrophosphatase-I from  HB8 (
HB8 ( ADPRase-I) prevents the intracellular accumulation of ADP-ribose by hydrolyzing it to AMP and ribose 5'-phosphate. To understand the catalytic mechanism of
ADPRase-I) prevents the intracellular accumulation of ADP-ribose by hydrolyzing it to AMP and ribose 5'-phosphate. To understand the catalytic mechanism of  ADPRase-I, it is necessary to investigate the role of glutamates and metal ions as well as the coordination of water molecules located at the active site. A macroseeding method was developed in order to obtain a large
ADPRase-I, it is necessary to investigate the role of glutamates and metal ions as well as the coordination of water molecules located at the active site. A macroseeding method was developed in order to obtain a large  ADPRase-I crystal which was suitable for a neutron diffraction study to provide structural information. Neutron and X-ray diffraction experiments were performed at room temperature using the same crystal. The crystal diffracted to 2.1 and 1.5
ADPRase-I crystal which was suitable for a neutron diffraction study to provide structural information. Neutron and X-ray diffraction experiments were performed at room temperature using the same crystal. The crystal diffracted to 2.1 and 1.5  resolution in the neutron and X-ray diffraction experiments, respectively. The crystal belonged to the primitive space group
resolution in the neutron and X-ray diffraction experiments, respectively. The crystal belonged to the primitive space group  3
3 21, with unit-cell parameters
21, with unit-cell parameters 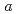 =
=  = 50.7,
= 50.7, 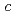 = 119
= 119  .
.