Synthesis of radioiodinated antitumor cyclic peptide, [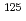 I]-sansalvamide A derivative
I]-sansalvamide A derivative
抗腫瘍効果を持つ放射性ヨウ素化環状ペプチド[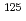 I]-sansalvamide A誘導体の合成
I]-sansalvamide A誘導体の合成
渡邉 茂樹; 山田 圭一*; 津久井 匠隆*; 花岡 宏史*; 大島 康宏; 山口 藍子*; 奥 浩之*; 石岡 典子
Watanabe, Shigeki; Yamada, Keiichi*; Tsukui, Narutaka*; Hanaoka, Hirofumi*; Ohshima, Yasuhiro; Yamaguchi, Aiko*; Oku, Hiroyuki*; Ishioka, Noriko
Sansalvamide A (SA), a penta cyclic peptide isolated marine fungus, is a lead compound of anti-cancer reagent because the peptide has cytotoxicity against various cancer cell lines. Halogenated SA derivatives (SA-X, X = Cl, Br, I) was prepared and remarkable cytotocity against malignant human breast cancer. In this study, a radiohalogenated SA derivative [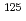 I]SA-I was prepared to conduct in vivo evaluation of SA derivatives. Synthetic scheme of [
I]SA-I was prepared to conduct in vivo evaluation of SA derivatives. Synthetic scheme of [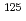 I]SA-I are as follows: an iodinated linear peptide, Boc-F(p-I)LLVL-OMe, was prepared by the conventional solid phase peptide synthesis. After preparation of stannylated peptide, Boc-F(p-SnBu
I]SA-I are as follows: an iodinated linear peptide, Boc-F(p-I)LLVL-OMe, was prepared by the conventional solid phase peptide synthesis. After preparation of stannylated peptide, Boc-F(p-SnBu )LLVL-OMe,
)LLVL-OMe, 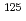 I was labeled with electrophilic destannylation in the presence of oxidizing reagent. After deprotection of N- and C-termius, [
I was labeled with electrophilic destannylation in the presence of oxidizing reagent. After deprotection of N- and C-termius, [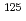 I]SA-I was obtained successfully by macrocyclization in liquid phase. Overall labeled yield was 7%. To our best knowledge, this report is the first on the synthesis of radiolabeled SA derivative. In vivo evaluation of the SA derivative using [
I]SA-I was obtained successfully by macrocyclization in liquid phase. Overall labeled yield was 7%. To our best knowledge, this report is the first on the synthesis of radiolabeled SA derivative. In vivo evaluation of the SA derivative using [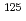 I]SA-I is being undertaken.
I]SA-I is being undertaken.