Spectroscopic study of Np(V) oxidation to Np(VI) in 3 mol/dm nitric acid at elevated temperatures
nitric acid at elevated temperatures
3mol/dm の加熱硝酸中におけるNp(V)のNp(VI)への酸化の分光学的研究
の加熱硝酸中におけるNp(V)のNp(VI)への酸化の分光学的研究
伴 康俊 
 ; 袴塚 保之; 筒井 菜緒
; 袴塚 保之; 筒井 菜緒 
 ; ト部 峻一; 萩谷 弘通; 松村 達郎
; ト部 峻一; 萩谷 弘通; 松村 達郎 

Ban, Yasutoshi; Hakamatsuka, Yasuyuki; Tsutsui, Nao; Urabe, Shunichi; Hagiya, Hiromichi; Matsumura, Tatsuro
加熱した3mol/dm 硝酸中におけるNpの吸光スペクトルを光路長1cmのウォータージャケット付き分光セルで測定し、Np(VI)のモル吸光係数(
硝酸中におけるNpの吸光スペクトルを光路長1cmのウォータージャケット付き分光セルで測定し、Np(VI)のモル吸光係数(
 )を種々の温度で求めた。
)を種々の温度で求めた。
 は温度の上昇と共に減少し、
は温度の上昇と共に減少し、
 の温度依存性を表す式として
の温度依存性を表す式として
 =-0.14
=-0.14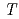 +85.5 (
+85.5 (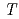 は温度)を得た。加熱した3mol/dm
は温度)を得た。加熱した3mol/dm 硝酸中におけるNp(V)のNp(VI)への酸化を上述の分光セルで観察し、Np(V)の酸化はNp(V)の濃度に対する疑一次反応として進行することを示した。さらに、336-362KにおけるNp(V)の酸化反応速度式として-d[Np(V)]
硝酸中におけるNp(V)のNp(VI)への酸化を上述の分光セルで観察し、Np(V)の酸化はNp(V)の濃度に対する疑一次反応として進行することを示した。さらに、336-362KにおけるNp(V)の酸化反応速度式として-d[Np(V)] /dt=2.2
/dt=2.2 10
10 exp[-65
exp[-65 10
10 /(
/(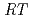 )][Np(V)]
)][Np(V)] (
( 及び[Np(V)]
及び[Np(V)] は気体定数及び時間
は気体定数及び時間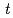 における[Np(V)]の濃度)を導出した。
における[Np(V)]の濃度)を導出した。
Optical absorption spectra of Np in 3 mol/dm nitric acid at elevated temperatures were measured using an optical glass cell with a water jacket having a light path of 1 cm, and molar extinction coefficients of Np(VI),
nitric acid at elevated temperatures were measured using an optical glass cell with a water jacket having a light path of 1 cm, and molar extinction coefficients of Np(VI), 
 , were obtained at various temperature. The values of
, were obtained at various temperature. The values of 
 was found to decrease with increasing the temperature, and could be described by the equation
was found to decrease with increasing the temperature, and could be described by the equation 
 = -0.14
= -0.14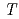 +85.5, where
+85.5, where 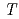 is the temperature. Oxidation of Np(V) to Np(VI) in 3 mol/dm
is the temperature. Oxidation of Np(V) to Np(VI) in 3 mol/dm nitric acid at elevated temperatures was observed using the optical glass cell. Oxidation of Np(V) proceeded as pseudo-first order with respect to Np(V) concentration. The rate equation in the temperature range of 336-362 K was obtained as follows: -d[Np(V)]
nitric acid at elevated temperatures was observed using the optical glass cell. Oxidation of Np(V) proceeded as pseudo-first order with respect to Np(V) concentration. The rate equation in the temperature range of 336-362 K was obtained as follows: -d[Np(V)] /dt = 2.2
/dt = 2.2 10
10 exp[-65
exp[-65 10
10 /(
/(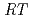 )][Np(V)]
)][Np(V)] , where
, where  and [Np(V)]
and [Np(V)] indicate the gas constant and Np(V) concentration at time
indicate the gas constant and Np(V) concentration at time 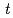 , respectively.
, respectively.