UO dissolution in bicarbonate solution with H
dissolution in bicarbonate solution with H O
O ; The Effect of temperature
; The Effect of temperature
H O
O による炭酸塩溶液中のUO
による炭酸塩溶液中のUO の溶解; 温度効果
の溶解; 温度効果
McGrady, J. 
 ; 熊谷 友多
; 熊谷 友多 

 ; 北辻 章浩
; 北辻 章浩 
 ; 桐島 陽*; 秋山 大輔*; 渡邉 雅之
; 桐島 陽*; 秋山 大輔*; 渡邉 雅之 


McGrady, J.; Kumagai, Yuta; Kitatsuji, Yoshihiro; Kirishima, Akira*; Akiyama, Daisuke*; Watanabe, Masayuki
使用済核燃料の埋設処分において、キャニスタが破損して核燃燃料が地下水と接触すると、水の放射線分解により発生した酸化性物質とUO が反応する。炭酸水素イオンが存在する地下水中での、H
が反応する。炭酸水素イオンが存在する地下水中での、H O
O によるUO
によるUO の酸化溶解がこれまでに研究されてきた。埋設されたキャニスタ周辺の温度は、時間や配置場所により変化するが、これまでに酸化溶解の温度効果は明らかにされていない。種々の炭酸水素イオン濃度と温度条件下でのUO
の酸化溶解がこれまでに研究されてきた。埋設されたキャニスタ周辺の温度は、時間や配置場所により変化するが、これまでに酸化溶解の温度効果は明らかにされていない。種々の炭酸水素イオン濃度と温度条件下でのUO 表面におけるH
表面におけるH O
O の反応とU溶解を調べた。炭酸水素イオン濃度や温度によりUO
の反応とU溶解を調べた。炭酸水素イオン濃度や温度によりUO 表面でのH
表面でのH O
O の反応機構が変化し、これによりUO
の反応機構が変化し、これによりUO の溶解速度が変化することが分かった。これは、UO
の溶解速度が変化することが分かった。これは、UO 表面に生成される化学種の違いに起因することが示唆された。
表面に生成される化学種の違いに起因することが示唆された。
Upon nuclear waste canister failure and contact of spent nuclear fuel with groundwater, the UO matrix of spent fuel will interact with oxidants in the groundwater generated by water radiolysis. Bicarbonate (HCO
matrix of spent fuel will interact with oxidants in the groundwater generated by water radiolysis. Bicarbonate (HCO
 ) is often found in groundwater, and the H
) is often found in groundwater, and the H O
O induced oxidative dissolution of UO
induced oxidative dissolution of UO in bicarbonate solution has previously been studied under various conditions. Temperatures in the repository at the time of canister failure will differ depending on the location, yet the effect of temperature on oxidative dissolution is unknown. To investigate, the decomposition rate of H
in bicarbonate solution has previously been studied under various conditions. Temperatures in the repository at the time of canister failure will differ depending on the location, yet the effect of temperature on oxidative dissolution is unknown. To investigate, the decomposition rate of H O
O at the UO
at the UO surface and dissolution of U
surface and dissolution of U in bicarbonate solution (0.1, 1, 10 and 50 mM) was analysed at various temperatures (10, 25, 45 and 60
in bicarbonate solution (0.1, 1, 10 and 50 mM) was analysed at various temperatures (10, 25, 45 and 60  C). At [HCO
C). At [HCO
 ]
] 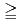 1 mM, the apparent equilibrium concentration of U
1 mM, the apparent equilibrium concentration of U decreased with increasing temperature. This was attributed to the formation of U
decreased with increasing temperature. This was attributed to the formation of U -bicarbonate species at the surface and a change in the mechanism of H
-bicarbonate species at the surface and a change in the mechanism of H O
O decomposition from oxidative to catalytic. At 0.1 mM, no obvious correlation between temperature and U dissolution was observed, and thermodynamic calculations indicated this was due to a change in the surface species. A pathway to explain the observed dissolution behaviour of UO
decomposition from oxidative to catalytic. At 0.1 mM, no obvious correlation between temperature and U dissolution was observed, and thermodynamic calculations indicated this was due to a change in the surface species. A pathway to explain the observed dissolution behaviour of UO in bicarbonate solution as a function of temperature was proposed.
in bicarbonate solution as a function of temperature was proposed.