Surface alteration of simulated nuclear fuel debris containing Fe, Cr, and Ni in water; A Raman and M ssbauer spectroscopic study
ssbauer spectroscopic study
ステンレス鋼成分を含む模擬デブリの水中での表面変質; ラマン分光法・メスバウアー分光法による研究
熊谷 友多 

 ; 日下 良二
; 日下 良二 
 ; 中田 正美
; 中田 正美 
 ; 渡邉 雅之
; 渡邉 雅之 

 ; 桐島 陽*; 秋山 大輔*; 佐藤 修彰*; 佐々木 隆之*
; 桐島 陽*; 秋山 大輔*; 佐藤 修彰*; 佐々木 隆之*
Kumagai, Yuta; Kusaka, Ryoji; Nakada, Masami; Watanabe, Masayuki; Kirishima, Akira*; Akiyama, Daisuke*; Sato, Nobuaki*; Sasaki, Takayuki*
Fuel debris generated in the Fukushima Daiichi NPS accident remains in the damaged reactors and substantial time and effort will be required until the retrieval of the debris. The debris is most likely contacted with water since the accident. The contact with water has possibility to induce degradation of the debris. According to the researches of uranium oxides and spent fuels, the uranium oxide matrix of fuels is oxidized and gradually dissolved as a consequence of water radiolysis. This oxidative dissolution process must be associated with surface alteration. In order to examine these possible degradation processes, we have conducted leaching experiments using simulated fuel debris combined with surface analysis by Raman spectroscopy and 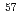 Fe M
Fe M ssbauer spectroscopy in backscatter geometry. The simulated fuel debris used in this study was prepared from powders of UO
ssbauer spectroscopy in backscatter geometry. The simulated fuel debris used in this study was prepared from powders of UO and stainless steel (1 : 1 in atomic ratio of U : Fe + Cr + Ni) by heat treatments. The samples were immersed in pure water or aqueous H
and stainless steel (1 : 1 in atomic ratio of U : Fe + Cr + Ni) by heat treatments. The samples were immersed in pure water or aqueous H O
O solution for up to 30 days. Aqueous H
solution for up to 30 days. Aqueous H O
O solution was used to simulate the water radiolysis. After certain periods of immersion, the samples were analyzed by Raman spectroscopy and M
solution was used to simulate the water radiolysis. After certain periods of immersion, the samples were analyzed by Raman spectroscopy and M ssbauer spectroscopy as well as chemical analysis of leached elements. The analysis of leached elements showed a selective dissolution of U from the samples. The U dissolution was facilitated by the reaction of H
ssbauer spectroscopy as well as chemical analysis of leached elements. The analysis of leached elements showed a selective dissolution of U from the samples. The U dissolution was facilitated by the reaction of H O
O . The reaction of H
. The reaction of H O
O also resulted in formation of solid uranyl peroxides, UO
also resulted in formation of solid uranyl peroxides, UO
 4H
4H O and UO
O and UO
 2H
2H O. The formation of uranyl peroxides on the surface was clearly confirmed by the Raman spectroscopy. The
O. The formation of uranyl peroxides on the surface was clearly confirmed by the Raman spectroscopy. The 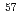 Fe M
Fe M ssbauer spectroscopy, in contrast, showed insignificant change between the spectra before and after the immersion. The result of
ssbauer spectroscopy, in contrast, showed insignificant change between the spectra before and after the immersion. The result of 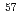 Fe M
Fe M ssbauer spectroscopy suggests that phases containing Fe are stable toward water and H
ssbauer spectroscopy suggests that phases containing Fe are stable toward water and H O
O .
.